These Indian-Made Cough Syrups Could Cause Acute Kidney Injuries To Your Kid.
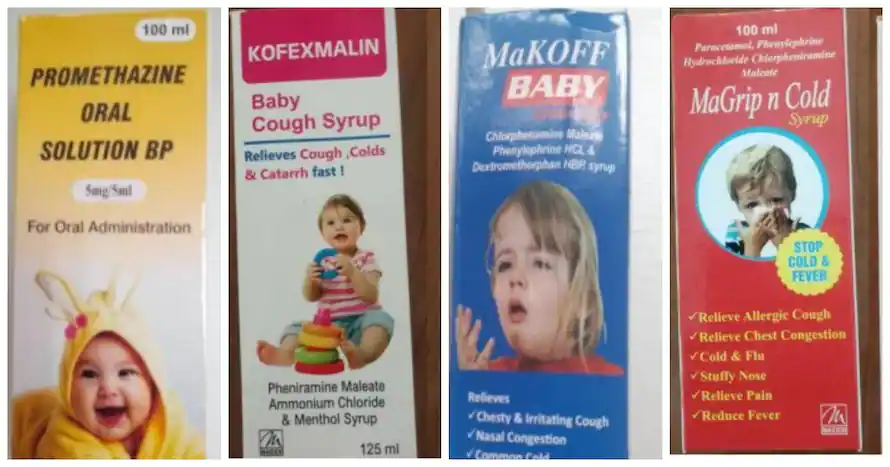

After the death of 66 children in the west African nation of The Gambia, the World Health Organization (WHO) issued an alert for four “contaminated” India-made cough and cold syrups, mainly prescribed for pediatric use. “WHO has today issued a medical product alert for four contaminated medicines identified in the Gambia that have been potentially linked with acute kidney injuries and 66 deaths among children. The loss of these young lives is beyond heartbreaking for their families,” the WHO said in a series of tweets, citing its Director-General Tedros Adhanom Ghebreyesus.
INDIAN-MADE COUGH SYRUPS THAT ARE BANNED BY WHO
In a statement, the WHO mentioned that all four ‘contaminated’ medicines are manufactured by Maiden Pharmaceuticals Ltd located in Haryana. The four cough and cold syrups that are banned by WHO are:-
- Promethazine Oral Solution
- Kofexmalin Baby Cough Syrup
- Makoff Baby Cough Syrup
- Magrip N Cold Syrup
“Laboratory analysis of samples of each of the four products confirms that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants,” the WHO said in a medical product alert.
WHAT ARE THE RISKS?
The WHO said that the syrups had diethylene glycol and ethylene glycol, which are toxic and can be fatal. These ingredients can cause:
- Abdominal pain
- Vomiting
- Diarrhoea
- Headache
- Severe renal injury
WHO’S ADVICE TO PUBLIC
“Please DO NOT use them. It is important to detect and remove these substandard products from circulation to prevent harm to patients,” said the WHO in a statement. Furthermore, it urged people to seek immediate medical consultation from a licensed healthcare provider if they or someone they know has used these items or had any negative side effects.