Why These 4 Indian Cough Syrups Banned by WHO Could be Dangerous: They Contain a Toxic Chemical
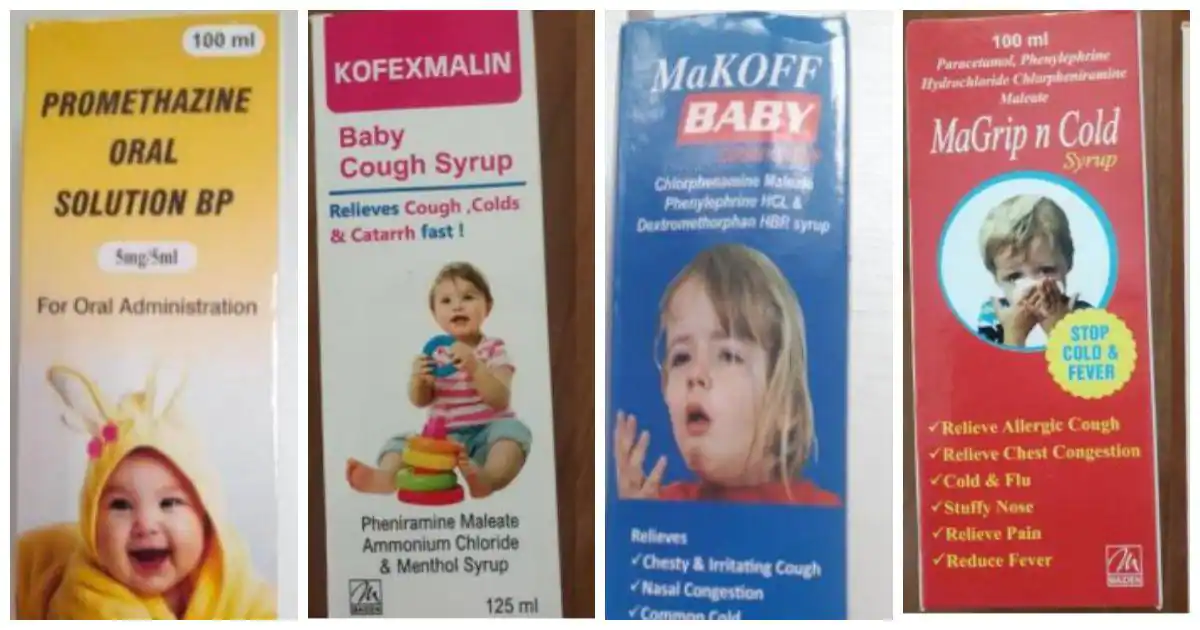

Cough Syrups by Maiden Pharmaceuticals Banned: Soon after the World Health Organization (WHO) banned four India-made cough and cold syrups, the Central Drugs Standard Control Organisation (CDSCO) launched a probe over the drugs linked to the death of 66 children in The Gambia. Manufactured by Haryana-based Maiden Pharmaceuticals Ltd, the four cough and cold syrups that are banned by WHO are —Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup.
“The four medicines are cough and cold syrups produced by Maiden Pharmaceuticals Limited, in India. They failed the test as they have unacceptable amounts of diethylene glycol and ethylene glycol as contaminants”, the global health agency had stated. According to the tentative results received by the WHO, four out of the 23 samples tested have been found to contain either Diethylene Glycol/Ethylene Glycol.
WHAT IS DIETHYLENE GLYCOL, WHY IS IT DANGEROUS?
- Diethylene Glycol or Ethylene Glycol is considered toxic for human body. When consumed, it can result in kidney and neurological toxicity. DEG has been associated with several cases of mass poisoning when consumed via drugs.
- It is a colorless, practically odorless, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol.
- The toxic effects of Diethylene Glycol include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury.
- According to a paper in the National Library of Medicine 10 DEG mass poisonings have occurred over the past 70 years. These mass poisonings were all caused by DEG-contaminated liquid or ointment medications. DEG contamination occurs when it is used in medicinal products instead of a safer—but more expensive—diluent such as pharmaceutical grade glycerin.
CDSCO INVESTIGATION UNDERWAY
Meanwhile, sources close to the matter revealed that the CDSCO has taken up the matter and ordered a detailed investigation against the cough and cold syrups produced and exported to The Gambia by Maiden Pharmaceuticals Limited.
The preliminary enquiry has revealed that Maiden Pharmaceutical Limited is a manufacturer licensed by the State Drug Controller for the products under reference, and holds manufacturing permission for these products.